Tuneable single-molecule electronic conductance of C60 by encapsulation†
Abstract
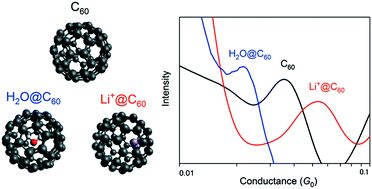
It has been demonstrated that the single-molecule transport properties of fullerene C60 can be modulated by encapsulating endohedral species, i.e. Li+ and H2O, which exhibit different degrees of van der Waals interactions with the C60 cage. Single-molecule junctions were prepared between the gaps of Au electrodes using a break junction technique. Encapsulation of H2O inside the cage caused a slight decrease in the electronic conductivity relative to that of pristine C60. This is in sharp contrast to Li+ encapsulation, which results in a twofold-to-fourfold increase in the conductivity. The electronic couplings between the C60 cage and the Au electrodes were weakly dependent on the endohedral species in the cage, though the molecular orbital energy levels were remarkably modulated upon encapsulation.


 Please wait while we load your content...
Please wait while we load your content...