Chemical equilibria of aqueous ammonium–carboxylate systems in aqueous bulk, close to and at the water–air interface†
Abstract
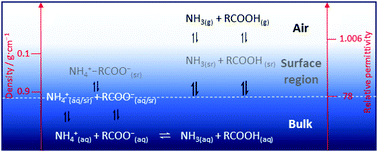
Previous studies have shown that the water–air interface and a number of water molecule layers just below it, the surface region, have significantly different physico-chemical properties, such as lower relative permittivity and density, than bulk water. The properties in the surface region of water favor weakly hydrated species as neutral molecules, while ions requiring strong hydration and shielding of their charge are disfavored. In this study the equilibria NH4+(aq) + RCOO−(aq) ⇌ NH3(aq) + RCOOH(aq) are investigated for R = CnH2n+1, n = 0–8, as open systems, where ammonia and small carboxylic acids in the gas phase above the water surface are removed from the system by a gentle controlled flow of nitrogen to mimic the transport of volatile compounds from water droplets into air. It is shown that this non-equilibrium transport of chemicals can be sufficiently large to cause a change of the chemical content of the aqueous bulk. Furthermore, X-ray photoelectron spectroscopy (XPS) has been used to determine the relative concentration of alkyl carboxylic acids and their conjugated alkyl carboxylates in aqueous surfaces using a micro-jet. These studies confirm that neutral alkyl carboxylic acids are accumulated in the surface region, while charged species, as alkyl carboxylates, are depleted. The XPS studies show also that the hydrophobic alkyl chains are oriented upwards into regions with lower relative permittivity and density, thus perpendicular to the aqueous surface. These combined results show that there are several chemical equilibria between the aqueous bulk and the surface region. The analytical studies show that the release of mainly ammonia is dependent on its concentration in the surface region, as long as the solubility of the carboxylic acid in the surface region is sufficiently high to avoid a precipitation in/on the water–air interface. However, for n-octyl- and n-nonylcarboxylic acid the solubility is sufficiently low to cause precipitation. The combined analytical and surface speciation studies in this work show that the equilibria involving the surface region are fast. The results from this study increase the knowledge about the distribution of chemical species in the surface region at and close to the water–air interface, and the transport of chemicals from water to air in open systems.


 Please wait while we load your content...
Please wait while we load your content...