The prediction of high-pressure volumetric properties of compressed liquids using the two states model†
Abstract
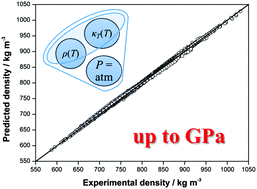
In this work, we argue that the volumetric properties of liquids cannot be reproduced by a single isothermal equation of state derived by the compressibility route for the whole pressure region extended up to a GPa pressure but require the consideration of two states associated with qualitatively different molecular packing properties. This is confirmed by examples of polar and non-polar substances within the range of temperatures from 203.15 K to 491.48 K and pressures up to 1200 MPa. The proposed two states model is truly predictive for the high-pressure density and isothermal compressibility using several easily measurable physico-chemical quantities: the density, the isobaric heat capacity, and the speed of sound at atmospheric pressure only. The experimental data on the density for 15 different compressed liquids, given in the literature as a function of temperature and very high-pressures, were used for the comparison and its analysis. The relative absolute average deviation for 2138 experimental data points by a two states model is close to 0.17%.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...