Formation of a stable biradical triplet state cation versus a closed shell singlet state cation by oxidation of adducts of 3,6-dimethoxycarbazole and polychlorotriphenylmethyl radicals†
Abstract
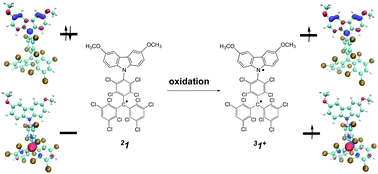
We report an experimental and theoretical study of two stable radical adducts of the triphenylmethyl series, 1 and 2, whose composition and molecular structure are distinguished by the content and position of chlorine atoms in phenyls. The electrochemical study through cyclic voltammetry of these open layer species shows the existence of two reversible processes, related to reduction and oxidation, to stable charged species. The chemical oxidation of both radical adducts gives rise to stable cations, whose fundamental state has a biradical triplet electronic structure or a closed shell singlet character, depending on the electronic conjugation between the donor and acceptor electron moieties. The presence of chlorines adjacent to the nitrogen in 1 breaks the conjugation between both halves, facilitating the formation of a triplet electronic state of the cation, while the absence of chlorines in these positions in 2 facilitates partial conjugation and stabilizes the closed shell singlet electronic state of the cation.


 Please wait while we load your content...
Please wait while we load your content...