Osmolytes modify protein dynamics and function of tetrameric lactate dehydrogenase upon pressurization†
Abstract
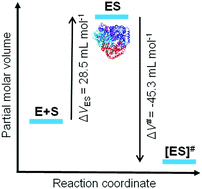
We present a study of the combined effects of natural cosolvents (TMAO, glycine, urea) and pressure on the activity of the tetrameric enzyme lactate dehydrogenase (LDH). To this end, high-pressure stopped-flow methodology in concert with fast UV/Vis spectroscopic detection of product formation was applied. To reveal possible pressure effects on the stability and dynamics of the enzyme, FTIR spectroscopic and neutron scattering measurements were carried out. In neat buffer solution, the catalytic turnover number of the enzyme, kcat, increases up to 1000 bar, the pressure range where dissociation of the tetrameric species to dimers sets in. Accordingly, we obtain a negative activation volume, ΔV# = −45.3 mL mol−1. Further, the enzyme substrate complex has a larger volume compared to the enzyme and substrate in the unbound state. The neutron scattering data show that changes in the fast internal dynamics of the enzyme are not responsible for the increase of kcat upon compression. Whereas the magnitude of kcat is similar in the presence of the osmolytes, the pressure of deactivation is modulated by the addition of cosolvents. TMAO and glycine increase the pressure of deactivation, and in accordance with the observed stabilizing effect both cosolvents exhibit against denaturation and/or dissociation of proteins. While urea does not markedly affect the magnitude of the Michaelis constant, KM, both 1 M TMAO and 1 M glycine exhibit smaller KM values of about 0.07 mM and 0.05 mM below about 1 kbar. Such positive effect on the substrate affinity could be rationalized by the effect the two cosolutes impose on the thermodynamic activities of the reactants, which reflect changes in water-mediated intermolecular interactions. Our data show that the intracellular milieu, i.e., the solution conditions that have evolved, may be sufficient to maintain enzymatic activity under extreme environmental conditions, including the whole pressure range encountered on Earth.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...