Understanding of transition metal (Ru, W) doping into Nb for improved thermodynamic stability and hydrogen permeability: density functional theory calculations
Abstract
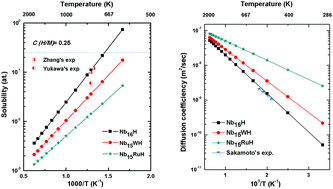
Hydrogen solubility and diffusivity are the key features of hydrogen permeable membrane materials. To characterize the hydrogen permeation performance of NbTM (TM = W, Ru) phases, their hydrogen diffusion coefficient and solution coefficient, thermodynamic stability and chemical bonding are studied by a series of first principles calculations. The phonon spectra and elastic constants show that NbTM is dynamically stable. The TM–H chemical bonds have an ionic/covalent mixed character and are stronger than the Nb–H bond. The preferential diffusion paths of H in both Nb16H and Nb15TMH are from a tetrahedral interstitial site (TIS) to another TIS. The TM doping in Nb16H lowers the solubility and energy barrier of H diffusion and enhances the H diffusion coefficient (D), with Nb16RuH exhibiting the highest D value for TIS to TIS diffusion (2.14 × 10−8 m2 s−1) at 600 K. This study shows that alloying and temperature could significantly affect the solubility and diffusivity of hydrogen in Nb. Moreover, TM doping could greatly improve the hydrogen diffusion performance with good control of hydrogen embrittlement.


 Please wait while we load your content...
Please wait while we load your content...