Ultrafast flavin/tryptophan radical pair kinetics in a magnetically sensitive artificial protein†
Abstract
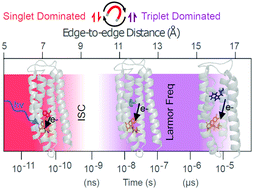
Radical pair formation and decay are implicated in a wide range of biological processes including avian magnetoreception. However, studying such biological radical pairs is complicated by both the complexity and relative fragility of natural systems. To resolve open questions about how natural flavin–amino acid radical pair systems are engineered, and to create new systems with novel properties, we developed a stable and highly adaptable de novo artificial protein system. These protein maquettes are designed with intentional simplicity and transparency to tolerate aggressive manipulations that are impractical or impossible in natural proteins. Here we characterize the ultrafast dynamics of a series of maquettes with differing electron-transfer distance between a covalently ligated flavin and a tryptophan in an environment free of other potential radical centers. We resolve the spectral signatures of the cysteine-ligated flavin singlet and triplet states and reveal the picosecond formation and recombination of singlet-born radical pairs. Magnetic field-sensitive triplet-born radical pair formation and recombination occurs at longer timescales. These results suggest that both triplet- and singlet-born radical pairs could be exploited as biological magnetic sensors.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...