Synergy of orientational relaxation between bound water and confined water in ice cold-crystallization†
Abstract
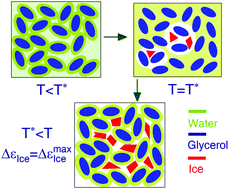
Cold-crystallization of systems with high glass-forming ability, such as medium-concentrated aqueous solutions, metallic glass formers and polymers, is a phenomenon of fundamental interest. In medium-concentrated aqueous solutions, bulk-like free water disappears and water comprises bound water and confined water surrounded by closely compacted hydrated solutes. Here, we used dielectric spectroscopic measurements of aqueous glycerol solutions to show that bound water and confined water participate in the cold-crystallization of water in a dynamically synergetic manner. Cold-crystallization of water begins when orientational relaxations of these two kinds of water are in concert. Notably, complete dehydration of some glycerol molecules occurs upon cold-crystallization of water, and these molecules become fully rehydrated just after this event. Hence, bound water is also crystallizable in intermediate concentrated glycerol solutions; although the same amount of water molecules as that of bound water do not eventually participate in cold-crystallization. This observation is significantly different from the traditionally suggested non-crystallization of bound water in dilute solutions. This work suggests the need to reevaluate the role of bound water in water crystallization, and might also reveal the mechanism of cold-crystallization occurring in metallic glass formers and polymers.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...