The impact of cation acidity and alkyl substituents on the cation–anion interactions of 1-alkyl-2,3-dimethylimidazolium ionic liquids†
Abstract
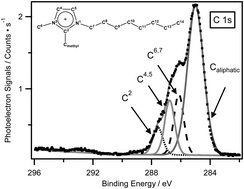
In this study, eight 1-alkyl-2,3-dimethylimidazolium ionic liquids are analysed by X-ray photoelectron spectroscopy. The effect of both the anion and the cation on the electronic environment of cationic nitrogen regions is explored. It concludes that the cationic N 1s binding energy shifts to the lower value when the basicity of the anion increases or the acidity of the cation decreases. The impact of the cation acidity on the cation–anion interactions is demonstrated systematically by carefully comparing the binding energies of anion-based components for each anion. It is found that for more basic anions, the charge-transfer effect between counterions can be effectively shielded; for less basic anions, such an effect is negligible. The charge shielding effect of the alkyl substituent is also studied by using dodecyl-based ionic liquids, compared to 1-alkyl-3-methylimidazolium analogues. It suggests that long alkyl substituents can have a significant electron donating effect to the cation headgroup and thus effectively shield the charge-transfer effect between cations and anions.


 Please wait while we load your content...
Please wait while we load your content...