Determining the gas composition for the growth of BNNTs using a thermodynamic approach
Abstract
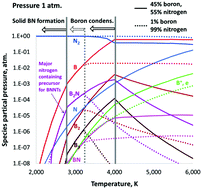
High-yield production of high-quality boron-nitride nanotubes (BNNTs) has been reported recently in several publications. A boron-rich material is evaporated using a laser or plasma in a nitrogen-rich atmosphere to supply precursor gaseous species for nucleation and growth of BNNTs. Either hydrogen was added or pressure was increased in the system to achieve high yield and high purity of the synthesized nanotubes. According to the widely-accepted “root grow” mechanism, upon gas cooling, boron droplets form first, then they adsorb nitrogen from the surrounding gas species, and BNNTs grow on their surfaces. However, what are the precursor species that provide nitrogen for the growth is still an open question. To answer this question, we performed thermodynamic calculations for determining the B–N mixture composition considering a broad set of gas species. For the first time, condensation of boron was taken into account and was shown to have a drastic effect on thegas chemical composition. B2N molecules were identified to be a major source of nitrogen for the growth of BNNTs. The presence of B2N molecules in a B–N gas mixture was verified by our spectroscopic measurements during laser ablation of boron-rich targets in nitrogen. It was shown that the increase of pressure has a quantitative effect on the mixture composition yielding an increase of the precursor density. Hydrogen addition might open an additional channel of nitrogen supply to support the growth of BNNTs. The nitrogen atoms react with abundant H2 molecules to form NH2 and then NH3 precursor species, instead of just recombining back to inert N2 molecules, as in the no-hydrogen case. In addition, thermodynamics was applied in conjunction with agglomeration theory to predict the size of the boron droplets upon growth of BNNTs. Analytical relations for the identification of crucial species densities were derived.


 Please wait while we load your content...
Please wait while we load your content...