Significance of hydrogen bonding networks in the proton-coupled electron transfer reactions of photosystem II from a quantum-mechanics perspective†
Abstract
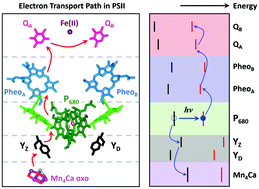
The photosynthetic protein complex, photosystem II (PSII), conducts the light-driven water-splitting reaction with unrivaled efficiency. Proton-coupled electron transfer (PCET) reactions at the redox-active tyrosine residues are thought to play a critical role in the water-splitting chemistry. Addressing the fundamental question as to why the tyrosine residue, YZ, is kinetically competent in comparison to a symmetrically placed tyrosine residue, YD, is important for the elucidation of the mechanism of PCET in the water-splitting reaction of PSII. Here, using all-quantum-mechanical calculations we study PCET at the YZ and YD residues of PSII. We find that when YZ is in its protein matrix under physiological conditions, the HOMO of YZ constitutes the HOMO of the whole system. In contrast, the HOMO of YD is buried under the electronic states localized elsewhere in the protein matrix and PCET at YD requires the transfer of the phenolic proton, which elevates the HOMO of YD to become the HOMO of the whole system. This leads to the oxidation of YD, albeit on a slower timescale. Our study reveals that the key differences between the electronic structure of YZ and YD are primarily determined by the protonation state of the respective hydrogen-bonding partners, D1-His190 and D2-His189, or more generally by the H-bonding network of the protein matrix.


 Please wait while we load your content...
Please wait while we load your content...