Formation of the quasi-planar B50 boron cluster: topological path from B10 and disk aromaticity†
Abstract
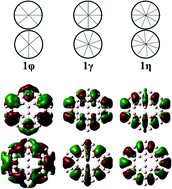
The lowest-lying isomer of the B50 boron cluster is confirmed to have a quasi-planar shape with two hexagonal holes. By applying a topological (leap-frog) dual operation followed by boron capping, we demonstrated that such a quasi-planar structure actually comes from the smallest elongated B102−, and its high thermodynamic stability is due to its inherent disk aromaticity arising from its 32 valent π electrons that fully occupy a disk configuration of [(1σ)2(1π)4(1δ)4(2σ)2(1φ)4(2π)4(1γ)4(2δ)4(1η)4]. The aromatic character of the quasi-planar B50 is further supported by a strong diatropic magnetic current flow. The sudden appearance of a quasi-planar B50 again points out that the growth pattern of pure boron clusters is still far from being completely understood.


 Please wait while we load your content...
Please wait while we load your content...