Insight into the internal structure of amyloid-β oligomers by isotope-edited Fourier transform infrared spectroscopy†
Abstract
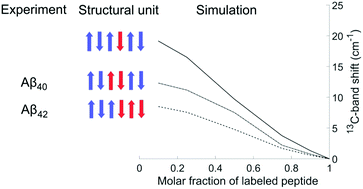
The internal structure of amyloid-β (Aβ) oligomers was investigated with isotope-edited Fourier transform infrared spectroscopy. Homo-oligomers of Aβ40 and Aβ42 were prepared from unlabeled and 13C, 15N-labeled monomeric Aβ and from mixtures of these. For the unlabeled peptides, two main bands were observed in 2H2O at 1685 and 1622 cm−1 for Aβ40 and at 1685 and 1626 cm−1 for Aβ42. These band positions indicate that the number of strands per sheet is at least four. The obtained experimental amide I spectra were simulated using a number of structural models (antiparallel β-sheets, β-barrels and a dodecamer structure). According to experiments and calculations, the main 13C-band shifts down at increasing molar ratio of labeled peptides. This shift occurs when vibrational coupling becomes possible between 13C-amide groups in close-by strands. It is small, when intervening 12C-strands increase the distance between 13C-strands; it is large, when many neighboring strands are labeled. The shift depends on the internal structure of the peptides within the oligomers, i.e. on the building block that each peptide molecule contributes to the β-sheets of the oligomers. The shift is largest, when individual peptides contribute just a single strand surrounded by strands from other peptide molecules. It is smaller when each molecule forms two or three adjacent strands. As indicated by a comparison between experiment and computation, the number of adjacent β-strands per peptide molecule is two for Aβ40 oligomers and two or more for Aβ42 oligomers. Our results are well explained by regular, antiparallel β-sheets or β-barrels.


 Please wait while we load your content...
Please wait while we load your content...