Conformational isomers of trans-urocanic acid observed by rotational spectroscopy†
Abstract
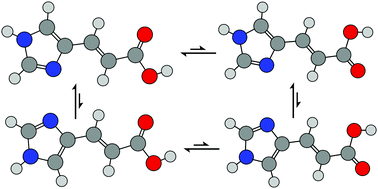
Rotational spectra have been measured and assigned for four conformers of trans-urocanic acid. The acid was transferred into the gas phase through laser vaporisation of a solid sample, mixed with a neon buffer gas and then cooled through supersonic expansion. Molecules and complexes in the expanding gas jet were probed through chirped-pulse, Fourier transform microwave spectroscopy between 2.0 and 18.5 GHz. Rotational constants, A0, B0 and C0; centrifugal distortion constants, ΔJ and ΔJK; and nuclear quadrupole coupling constants of the nitrogen atoms, χaa(N) and χbb(N)–χcc(N), were determined for the various conformers. Data were obtained for ten isotopologues of the conformer that was observed to yield the spectrum of highest intensity. Substitution (rs) coordinates were determined for all carbon atoms and two hydrogen atoms of this conformer. Other observed spectra were assigned to conformers on the basis of excellent agreement between calculated and experimentally-determined rotational constants, and empirical observations of the relative intensities of a- and b-type transitions. The results of DFT calculations imply high barriers to the interconversion of assigned conformers.


 Please wait while we load your content...
Please wait while we load your content...