Shape adaptation of quinine in cyclodextrin cavities: NMR studies†
Abstract
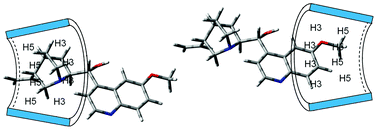
Complex formation between quinine and natural cyclodextrins (CD) was studied using NMR spectroscopy. The strongest association was observed for complexes of neutral quinine molecules with βCD. Association constants for monocationic quinine were one order of magnitude smaller, while dicationic quinine did not bind to CDs. The distribution of complexation-induced shifts and ROESY spectra revealed bimodal quinine binding in complexes formed with βCD and γCD. Complex formation resulted in a decrease of the vicinal coupling constant between H2 and H9 protons owing to the rotation about the C2−C9 bond and in consequence in mutual reorientation of two main constituents of quinine: quinoline and quinuclidine. DFT calculations allowed establishing that H2 and H9 protons are antiperiplanar in the prevailing quinine conformer(s) in aqueous solution. Conformers with synclinal H2 and H9 protons participated in quinine complexation with CDs.


 Please wait while we load your content...
Please wait while we load your content...