Photophysical properties of structural isomers of homoleptic Ir-complexes derived from xylenyl-substituted N-heterocyclic carbene ligands†
Abstract
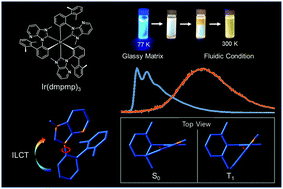
The phosphorescence properties of fac-Ir(pmp)3, mer-Ir(pmp)3, fac-Ir(dmpmp)3 and mer-Ir(dmpmp)3 (where pmp = 3-methyl-1-phenyl-2,3-dihydro-1H-imidazo[4,5-b]pyridine and dmpmp = 1-(2′,6′-dimethylbiphenyl-2-yl)-3-methyl-2,3-dihydro-1H-imidazo[4,5-b]pyridine) in CH2Cl2 were investigated. At 77 K, the fac-isomers showed blue emission with a vibronic structure, while the mer-isomers showed less structured emissions. At 300 K, all complexes showed broad and markedly red-shifted emission spectra compared to those at 77 K. The quantum yields of the Ir(dmpmp)3 isomers were very low, and their emission lifetimes were very short compared to those of Ir(pmp)3. In order to understand the large differences between the photodynamic properties of Ir(pmp)3 and Ir(dmpmp)3, we performed femtosecond time-resolved transient absorption (TA) spectroscopic measurements. The TA spectra of Ir(dmpmp)3 were almost the same as those of Ir(pmp)3 at a short delay time. However, Ir(dmpmp)3 showed a new broad TA band at around 720 nm with increasing delay time. The rise time of this band was ca. 10 ps for both isomers, and this may be attributed to the geometrical change in the excited state, which is associated with the steric hindrance of the bulky dimethylphenyl substituent. Actually, Ir(dmpmp)3 showed a strong rigidochromic shift in the emission spectra with varying temperature. To understand the molecular orbitals and the energy levels, theoretical calculations were performed using density functional theory. As a result, structural displacement takes place accompanied by the fast migration of localization of excited states via intraligand charge transfer.


 Please wait while we load your content...
Please wait while we load your content...