On the formation of phosphorous polycyclic aromatics hydrocarbons (PAPHs) in astrophysical environments†
Abstract
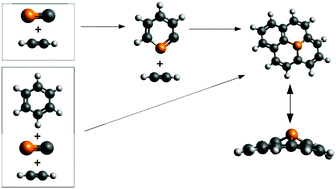
The formation of phosphorous-containing polycyclic aromatic hydrocarbons (PAPHs) in astrophysical contexts is proposed and analyzed by means of computational methods [B3LYP-D3BJ/ma-def2-TZVPP, MP2-F12, CCSD-F12b and CCSD(T)-F12b levels of theory]. A “bottom-up” approach based on a radical-neutral reaction scheme between acetylene (C2H2) and the CP radical was used investigating: (a) the synthesis of the first PAPH (C5H5P) “phosphinine”; (b) PAPH growth by addition of C2H2 to the C5H4P radical; (c) PAPH synthesis by addition reactions of one CP radical and nC2H2 to a neutral PAH. Results show: (I) the formation of the phosphinine radical has a strong thermodynamic tendency (−133.3 kcal mol−1) and kinetic barriers ≤5.4 kcal mol−1; (II) PAPH growth by nC2H2 addition on the radical phosphinine easily and exothermically produces radicals (1a- or 1-phospha-naphtalenes with kinetic barriers ≤7.1 kcal mol−1 and reaction free energies ≤−102.5 kcal mol−1); (III) the addition of a single CP + nC2H2 to a neutral benzene generates a complex chemistry where the main product is 2-phospha-naphtalene; (IV) because of the CP radical character, its barrierless addition to a PAH produces a resonant stabilized PAPH, becoming excellent candidates for addition reactions with neutral or radical hydrocarbons and PAHs; (V) the same energy trend between all four levels of theory continues a well-calibrated computational protocol to analyze complex organic reactions with astrochemical interest using electronic structure theory.


 Please wait while we load your content...
Please wait while we load your content...