Difference between approximate and rigorously measured transference numbers in fluorinated electrolytes†
Abstract
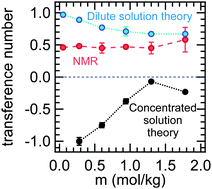
The performance of binary electrolytes is governed by three transport properties: conductivity, salt diffusion coefficient, and transference number. Rigorous methods for measuring conductivity and the salt diffusion coefficient are well established and used routinely in the literature. The commonly used methods for measuring transference number are the steady-state current method, t+,id, and pulsed field gradient NMR, t+,NMR. These methods yield the transference number only if the electrolyte is ideal, i.e., the salt dissociates completely into non-interacting anions and cations. In this work, we present a complete set of ion transport properties for mixtures of a functionalized perfluoroether, dimethyl carbonate terminated perfluorinated tetraethylene ether, and lithium bis(fluorosulfonyl)imide (LiFSI). The equations used to determine these properties from experimental data are based on Newman's concentrated solution theory. The concentrated-solution-theory-based transference number, t0+, is negative across all salt concentrations, and it increases with increasing salt concentration. In contrast, the ideal transference number, t+,id, is positive across all salt concentrations and it decreases with salt concentration. The NMR-based transference number, t+,NMR, is approximately 0.5, independent of salt concentration. The disparity between the three transference numbers, which indicates the dominance of ion clustering, is resolved by the use of Newman's concentrated solution theory.


 Please wait while we load your content...
Please wait while we load your content...