Shape-selective synthesis of nanoceria for degradation of paraoxon as a chemical warfare simulant†
Abstract
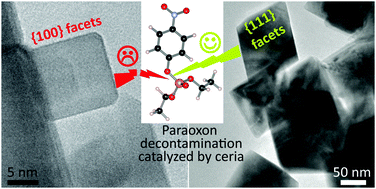
Repeated attacks using organophosphorus compounds, in military conflicts or terrorist acts, necessitate developing inexpensive and readily available decontamination systems. Nanosized cerium oxide is a suitable candidate, acting as a heterogeneous catalyst for the degradation of organophosphorus compounds such as VX agent or sarin. However, the reaction mechanism of the phosphatase mimetic activity of CeO2 nanoparticles is not fully described. Adsorption, surface-promoted hydrolysis, and desorption cycles strongly depend on the physico-chemical characteristics of the facets. In this study, CeO2 nanoparticles with different shapes were elaborated by hydrothermal synthesis. Nano-octahedra, nanocubes, or nanorods were selectively obtained under different conditions (temperature, concentration and nature of the precursors). The degradation activity according to the crystal faces was evaluated in vitro by measuring the degradation kinetics of paraoxon organophosphate in the presence of CeO2 nanoparticles. The results show an influence of both specific surface area and crystal faces of the nanoparticles, with higher activity for {111} facets compared to {100} facets at 32 °C. The relative activity between the facets is ascribed to the adsorption probability, assuming coordination between the phosphoryl oxygen and cerium atoms, but also to the surface density of the Ce doublets with relevant spacing for phosphatase mimetic activity.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...