Insights into the biotransformation of 2,4,6-trinitrotoluene by the old yellow enzyme family of flavoproteins. A computational study†
Abstract
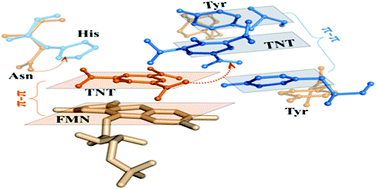
Biodegradation is a cost-effective and environmentally friendly alternative to removing 2,4,6-trinitrotoluene (TNT) pollution. However, mechanisms of TNT biodegradation have been elusive. To enhance the understanding of TNT biotransformation by the Old Yellow Enzyme (OYE) family, we investigated the crucial first-step hydrogen-transfer reaction by molecular dynamics simulations, docking technologies and empirical valence bond calculations. We revealed the significance of the π–π stacking conformation between the substrate TNT and the reduced flavin mononucleotide (FMNH2) cofactor, which is a prerequisite for the aromatic ring reduction of TNT. Under the π–π stacking conformation, the barrier of the hydrogen-transfer reaction in the aromatic ring reduction is about 16 kcal mol−1 lower than that of nitro group reduction. Then, we confirmed the mechanism of controlling the π–π stacking, that is, the π–π interaction competition mechanism. It indicates that the π–π stacking of TNT and FMNH2 occurs only when the π–π interaction between FMNH2 and TNT is stronger than that between TNT and several key residues with aromatic rings. Finally, based on the competition mechanism, the formation of π–π stacking of TNT and FMNH2 can be successfully enabled by removing the aromatic ring of those key residues in enzymes that originally only transform TNT through the nitro group reduction. This testified the validity of the π–π interaction competition mechanism. This work theoretically clarifies the molecular mechanism of the first-step hydrogen-transfer reaction for the biotransformation of TNT by the OYE family. It is helpful to obtain the enzymes that can biodegrade TNT through the aromatic ring reduction.


 Please wait while we load your content...
Please wait while we load your content...