On the large apparent Stokes shift of phthalimides
Abstract
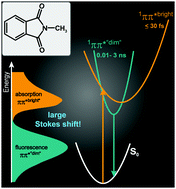
The photophysics of N-methylphthalimide (MP) in solution (cyclohexane, ethanol, acetonitrile, and water) was characterized by steady state as well as time resolved fluorescence and absorption spectroscopy. In all solvents the compound exhibits an unusually large Stokes shift of ∼10 000 cm−1. It is attributed to an ultrafast (<100 fs) depletion of the initially excited state, which results in the population of a weakly emitting state. Quantum chemical computations (DFT-MRCI) support this. They identify two energetically low-lying singlet ππ* excitations of different oscillator strength. Whereas the Stokes shift and thereby the ultrafast depletion of the initial excitation are hardly affected by the solvent later processes respond strongly. The fluorescence lifetime varies from ∼10 ps (cyclohexane) to ∼3 ns (water). This is attributed to a varying energetic accessibility of nπ* excitations.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...