Peroxy self-reaction leading to the formation of furfural†
Abstract
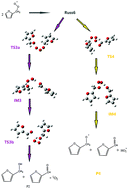
Interest in alternative fuels to petroleum and classical fuels has been growing very rapidly in recent years. Furan and its alkyl derivatives, such as methylfuran (2MF), have been identified as valid alternative biofuels. This study focuses on the self-reaction of the peroxy radical generated in the first oxidation step of 2MF, initiated by Cl atoms at 323 K and 4 Torr. The experiments have been carried out by a multiplexed synchrotron photoionization mass spectrometer (mSPIMS) at the Advanced Light Source (ALS) of Lawrence Berkeley National Laboratory (USA). The presence of a peak at m/z = 96 reveals that furfural is the dominant product of 2MF oxidation. Various reaction mechanisms for furfural formation are proposed here. The potential energy surfaces for singlet and triplet spin states have been mapped using quantum mechanical methods, such as CCSD(T), DFT-B3LYP, and composites models (CBS-QB3), to optimize the products, transition states, and intermediates. Experimental and theoretical results provide evidence that furfural does not form by primary reaction chemistry. Self-reaction of the peroxy radical generated in the first oxidation step of 2MF has been proposed as the pathway leading to the formation of furfural. Among various reaction channels, we indentified some entirely exothermic pathways involving oxygen–oxygen coupling and the formation of ROOOOR Russell intermediates.


 Please wait while we load your content...
Please wait while we load your content...