Swelling of clay minerals: dual characteristics of K+ ions and exploration of critical influencing factors†
Abstract
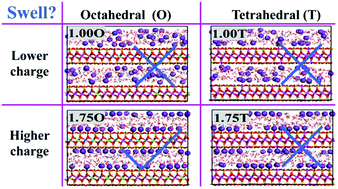
Clay swelling occurs frequently and is closely relevant to a number of engineering and industrial processes, while the underlying mechanisms remain elusive. In this study, K+-bearing clay systems with different charge amounts and charge locations have been simulated by molecular dynamics, showing that swelling is unfavorable for lower charge amounts (1.00 and 1.25 e uc−1) while it relies on charge locations for higher charge amounts (1.50 and 1.75 e uc−1): inhibited when tetrahedrally charged and favored when octahedrally charged. Accordingly, K+ shows dual characteristics and is not always a swelling inhibitor as generally thought. The various influencing factors are inspected and only the hydration effect interprets satisfactorily the swelling behaviors for all K+-bearing clay systems. The critical role of hydration effect during clay swelling is corroborated by the results of residence time, distribution of interlayer water and divergent swelling behaviors from Na+-bearing clay systems. Although water participates in a wide spectrum of physical and chemical processes, hydration is not necessarily among the most important influencing factors. Hydration effect has been evidenced as critical for clay swelling, and the results provide new insights into unraveling the complex swelling processes and resolving the associated engineering and industrial problems.


 Please wait while we load your content...
Please wait while we load your content...