Electrochemical properties of fluorinated boron-doped diamond electrodes via fluorine-containing plasma treatment†
Abstract
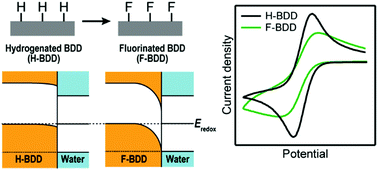
Since a boron-doped diamond (BDD) exhibits excellent electrode properties such as wide potential window, low back-ground current, and high physical and chemical durability, it has been studied as an electrode material for various electrochemical applications. The electrochemical behavior of BDD depends on the surface termination, which can be easily converted by chemical reactions. Fluorine termination has attracted interest because it exhibits unique surface properties such as high hydrophobicity and a low coefficient of friction, and the electrochemical properties also drastically change. However, so far, it has not been elucidated why fluorinated BDD exhibits specific electrochemical properties. In this article, fluorine-terminated BDD was fabricated by a fluorine-containing plasma treatment, and the electrochemical properties were systematically investigated. Together with experiments, we have calculated the interfacial structures and electronic states of hydrogenated, oxygenated, and fluorinated BDD electrodes. As a result, fluorinated BDD showed lower electrochemical reactivity than hydrogenated and oxygenated BDD. Especially, electron transfer between anionic compound and fluorinated BDD was significantly suppressed. Considered together with theoretical calculations, this reactivity could be attributed to the larger interfacial band bending in fluorinated BDD and electrostatic interactions between BDD and redox species.


 Please wait while we load your content...
Please wait while we load your content...