Self-assembled structure and dynamics of imidazolium-based protic salts in water solution
Abstract
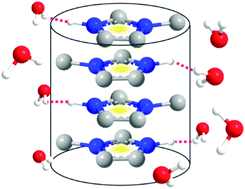
Protic ionic liquids containing cations with long alkyl chains can form self-assembled micelles, vesicles, microemulsions, and lyotropic liquid crystal structures in water, acid water or tetrahydrofuran, etc. As a result of this unique property, they are regarded as a novel category of amphiphiles, and are gaining importance in the field of colloid and interface chemistry. The critical micelle concentration (CMC) of protic salts, e.g., alkyl-ammonium nitrates in water, was found to increase with decreasing chain length. It is generally believed that a long alkyl chain length is essential for the formation of self-assembled structures. So far, no self-assembled structure has been reported for protic ionic liquids with an alkyl chain length of n < 4. This paper reports on the structure and dynamics of two imidazolium based protic organic salts with no alkyl chain or a methyl group (n = 1) attached to the cation in water solution, determined through a detailed analysis of NMR spectra and pulsed-field gradient NMR data. We demonstrate that these imidazolium cations with no or a short alkyl chain (n = 1) can form a self-assembled clustering structure in water solution, which has a strong influence on the diffusion behavior of imidazolium molecular ions. It is speculated that this self-assembled structure is likely to be present in other similar solutions of ionic liquids with short alkyl chains.


 Please wait while we load your content...
Please wait while we load your content...