Comment on “Decoding real space bonding descriptors in valence bond language” by A. Martín Pendás and E. Francisco, Phys. Chem. Chem. Phys., 2018, 20, 12368
Abstract
The authors of the above entitled paper suggest that molecular orbital (MO) and valence bond (VB) theories may generate conflicting insights into bonding. Therefore, they derive a real-space (RS) quantum chemical topology (QCT) approach (QCT-RS), and use it to extract insight into the H2 and LiH bonds, and contrast this insight with the one generated by VB calculations. The authors’ conclusions strongly contradict the usual bonding paradigms that arise from classical VB theory. Our Comment critically examines these claims and shows that MO and VB theories do not differ in their interpretations of bonding when both are applied on equal footing. It is furthermore shown that the conclusions based on this QCT-RS approach originate from a redefinition of VB structures in a manner that departs from the commonly accepted ones. This disparity of definitions of VB structures creates confusion. Thus, (a) the claim that in QCT-RS all covalency emanates from the covalent-ionic resonance, is generally incorrect in classical VB theory; and (b) contrary to the description of LiH as fully ionic in the above entitled paper, classical VB theory shows that this bond is well described as a superposition of a major covalent structure (albeit polarized) and a less important ionic one. The s–p hybridization of Li in the covalent structure is the major contributor to the dipole moment of LiH.
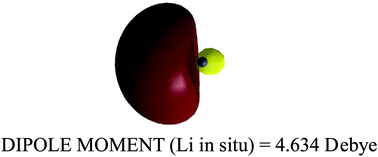


 Please wait while we load your content...
Please wait while we load your content...