Tritium trapping and migration mechanisms in Li2O: a first-principles study
Abstract
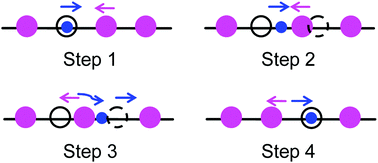
The tritium recovery behaviors and related mechanisms in lithium-based breeding materials are the major concerns in fusion reactors. In the present work, the energetics of intrinsic point defects and H-related defects in Li2O has been investigated by the first-principles method. The results show that the formation energies, charge states and relative stability of the intrinsic point defects and H-related defects in Li2O under working conditions vary with the energy level. Based on the defect properties and migration process, a model of the tritium trapping and migration mechanisms in Li2O is proposed: (1) the bred tritium is first trapped by oxygen vacancies; (2) subsequently the tritium detrapped from oxygen vacancies is retrapped by lithium vacancies, forming T substituents; and (3) the T substituents hop along the Li lattice.


 Please wait while we load your content...
Please wait while we load your content...