Infrared spectroscopic characterization of phosphate binding at the goethite–water interface†
Abstract
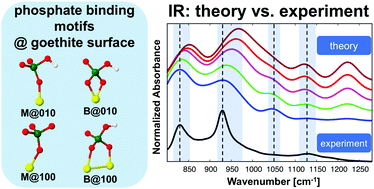
The interaction between phosphates and soil mineral surfaces, such as Fe- and Al-(oxyhydr)oxides, plays a crucial role in the immobilization of P and thus its availability for plants. The reactions of phosphates with Fe-hydroxides and especially goethite have been studied extensively. But a molecular-level picture of the phosphate binding mechanisms at the goethite–water interface is still lacking. Therefore, in the current contribution we have explored the molecular binding mechanisms for the adsorbed phosphate at the goethite–water interface by performing sorption kinetics experiments for orthophosphate and characterizing the adsorbed species by FT-IR spectroscopy. In parallel, periodic DFT calculations have been performed to explore the interaction mechanisms and to assign the IR spectra for monodentate (M) and bidentate (B) orthophosphate complexes at two different goethite surface planes (010 and 100) in the presence of water. In general, our interaction energy results give evidence that the mono-protonated B phosphate complex is favored to be formed at the goethite–water interface, although the M motif could exist as a minor fraction. Moreover, it was found that water plays an important role in controlling the phosphate adsorption process at the goethite surfaces. The interfacial water molecules form H-bonds (HBs) with the phosphate as well as with the goethite surface atoms. Furthermore, some water molecules form covalent bonds with goethite's Fe atoms while others dissociate at the surface to protons and hydroxyl groups. The present theoretical assignment of IR spectra introduces a benchmark for characterizing experimental IR data for the adsorbed KH2PO4 species at the goethite–water interface. In particular, the IR spectra of the mono-protonated (2O + 1Fe) B complex at the 010 goethite surface plane and the M complex at the 100 goethite surface plane were found to be consistent with the experimental data. In order to explore the role of different abundances of surface planes and binding motifs, IR spectra obtained from weighted averages have been analyzed. The results confirmed the conclusions drawn from interaction energy calculations.


 Please wait while we load your content...
Please wait while we load your content...