Theoretical studies on photo-induced cycloaddition and (6-4) reactions of the thymidine:4-thiothymidine dimer in a DNA duplex†
Abstract
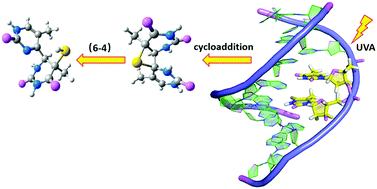
Thio-substituted nucleobases have received long-standing interest from experimental and theoretical scientists due to their potential applications in photodynamic therapy and crosslinking studies. Though the thymidine:4-thiothymidine dimer is an important structure in the DNA duplex, the molecular-level photoreaction mechanisms are still obscure. Herein, high-level QM/MM methods were adopted to investigate the photoinduced cycloaddition and (6-4) reactions of the thymidine:4-thiothymidine dimer in the DNA duplex, namely, d(ACCT(4ST)CGC:TGGAAGCG). Based on the calculated results, we identified five efficient nonadiabatic decay pathways to populate the T1 state from the initially occupied S2 state of Tp4ST via two crucial intersection structures, i.e., S2/S1 and S2/T2/S1/T1. Such photophysical processes are mainly localized on the 4-thiothymidine chromophore. After hopping to the T1 state, the light-induced [2+2] cycloaddition reaction could take place via a stepwise and nonadiabatic reaction pathway, which starts from Tp4ST via T1cc or T1cs intermediates in the T1 state and ends up with S5-thietane in the S0 state. By contrast, the concerted and thermal cycloaddition pathway in the ground state has a remarkable energy barrier, which is mechanistically less important. The subsequent generation of S5-(6-4) from S5-thietane is a concerted process in the S0 state with the simultaneous fission of the C4–S8 bond and the formation of the S8–H9 bond. In the end, we believe our present work will provide important mechanistic insights into photo-isomerization of thio-substituted nucleobases in DNA duplexes.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...