The effect of CO rotation from shaped pulse polarization on reactions that form C2†
Abstract
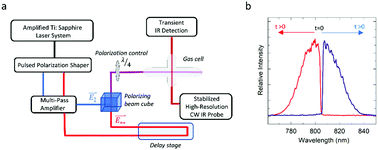
The effect of CO rotational energy on bimolecular reactions to form electronically excited C2 is reported here. The reactions are initiated by CO multiphoton absorption of 800 nm light in strong optical fields using two different polarization configurations based on shaped chirped pulses. The observation of Swan band emission indicates that C2(d3Πg) is a reaction product. The optical polarization is in the form of either an optical centrifuge or a dynamic polarization grating. In each case, the strong field aligns CO molecules and induces multiphoton absorption. Power-dependent measurements indicate at least seven photons are absorbed by CO; CO(a3Π) is a likely reactant candidate based on kinetic modeling. Relative reaction efficiencies are determined by measuring Swan band emission intensities. For a CO pressure of 100 Torr and an optical intensity of I = 2.0 × 1013 W cm−2, the relative C2(d3Πg) yield with the dynamic polarization grating is twice that with the optical centrifuge. The extent of CO rotational energy was determined for both optical polarizations using high-resolution transient IR absorption for a number of CO states with J = 62–73 and Erot up to 10 400 cm−1. Optical centrifuge excitation generates at least 2.5 times more rotationally excited CO molecules per quantum state than the dynamic polarization grating. The results indicate that the effect of large amounts of CO rotational energy is to reduce the yield of the C2 products.
- This article is part of the themed collections: 2019 PCCP HOT Articles and Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...