Possible B–C bonding in the hydroboration of benzonitrile by an external electric field†
Abstract
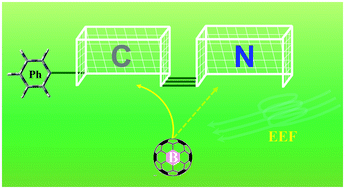
Generally, the hydroboration of benzonitrile produces B–N containing compounds. However, an unprecedented B–C bond may be formed in the presence of a suitable external electric field (EEF). In reactions of benzonitrile with borane, when the EEF is oriented parallel to the positive direction (N → C) of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond, the barriers to Markovnikov hydroboration are decreased gradually, meaning the pathway for generating B–C bonds becomes more favorable. Accordingly, hydroboration could be influenced and its selectivity could be controlled by changing the magnitude and direction of an applied EEF.
C bond, the barriers to Markovnikov hydroboration are decreased gradually, meaning the pathway for generating B–C bonds becomes more favorable. Accordingly, hydroboration could be influenced and its selectivity could be controlled by changing the magnitude and direction of an applied EEF.


 Please wait while we load your content...
Please wait while we load your content...