Surfactant-enhanced heterogeneity of the aqueous interface drives water extraction into organic solvents†
Abstract
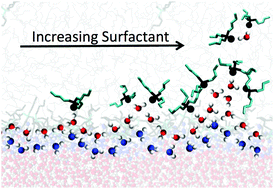
Liquid/liquid extraction (LLE) is one of the most industrially relevant separation methods, successfully leveraging the variable solubility of solutes (or their complexes) between two immiscible solvents. Independent of the relative solubilities of those solutes and complexes (which determine their distribution between phases), the dynamics of phase transfer processes are impacted by the molecular interactions and structure of those species at the interface. A simple example includes the formation and extraction of water–extractant adducts observed in the ternary water/organic/tri-n-butyl phosphate (TBP) system. Despite its implications for LLE, a detailed description of the structural and dynamic mechanisms by which such adducts are formed at the interface is not established. Describing that process requires connecting the evolving interfacial molecular organization in the presence of surfactants to dynamic surface fluctuations and interfacial heterogeneity. Herein, molecular dynamics simulation is combined with state-of-the-art network theory analysis to reveal features of interfacial structures and their relationship to the extraction of water in the water/n-hexane/TBP system. Surfactant adsorption enhances interfacial roughness which in turn causes directly interfacial water to become less connected through hydrogen bonding to subjacent layers, particularly upon formation of the water-bridged TBP dimer adduct. Furthermore, heterogeneity within the interface itself is enhanced by surfactant adsorption and serves as the basis for the formation of protrusions of water into the organic phase at the extremes of surface fluctuations. These features disproportionately incorporate the water-bridged TBP dimer and are the primary means by which water is transferred to the organic phase. This work presents for the first time a holistic understanding of how interfacial heterogeneity and spatial fluctuations become amplified in the presence of surfactants, enabling water extraction into the organic phase. It further affords the opportunity to study how solution conditions can control interfacial behavior to create more efficient solvent extraction systems.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...