Oxygen diffusion in amorphous and partially crystalline gallium oxide†
Abstract
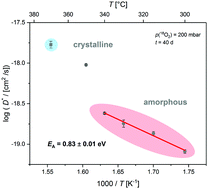
Oxygen transport in amorphous (a-GaO1.5) and partially crystalline (a/c-GaO1.5) gallium oxide was studied by means of 18O/16O isotope exchange experiments and time-of-flight secondary ion mass spectrometry (ToF-SIMS). Thin films of a-GaO1.5 were deposited by pulsed laser deposition (PLD) on alumina substrates at room temperature in an oxygen atmosphere. Oxygen tracer diffusion coefficients D* and oxygen surface exchange coefficients k* were determined as a function of temperature, 300 ≤ T/°C ≤ 370, and as a function of oxygen partial pressure, 2 ≤ p(O2)/mbar ≤ 500 at a temperature of T = 330 °C. The activation energy of oxygen tracer diffusion in amorphous gallium oxide was found to be EA = 0.8 eV. In addition, the time-temperature-transformation (TTT) diagram of crystallisation of amorphous gallium oxide was determined.


 Please wait while we load your content...
Please wait while we load your content...