The nature of binding of quinolate complex on the surface of ZnS quantum dots†
Abstract
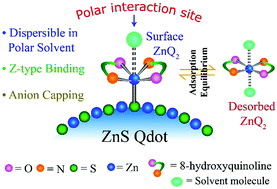
We report that the Z-type binding rather than X-type binding was favored when 8-hydroxyquinoline (HQ) reacted with presynthesized ZnS quantum dots (Qdots) to form surface zinc quinolinate complexes having a preferred stoichiometry of 1 : 2 (surface Zn2+ : HQ). Importantly, the higher solubility in polar solvents and high desorption coefficient (following Langmuir binding isotherm) of HQ-treated ZnS Qdot in DMSO solvent compared with those in methanol clearly indicated the favorable Z-type binding of HQ and thus the formation of surface octahedral ZnQ2 complex. Furthermore, the characteristics peaks in the 1H-nuclear magnetic resonance (NMR) spectrum of the desorbed species and the ligand density calculation of the surface complex (formed due to the reaction between HQ and ZnS Qdot) supported the octahedral ZnQ2 complex formation. Interestingly, the presence of dangling sulphide and the loss of planarity of ZnQ2 complex on the surface of ZnS Qdots (in turn gaining structural rigidity) may be the reasons for the Z-type binding of HQ. The specific binding might be the reason for superior optical properties and thermal stability of the surface ZnQ2 complex compared to the free ZnQ2 complex as such. The results can be considered important towards understanding the coordination chemistry of inorganic complex on the surface of Qdots and thus for their application potential.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...