Binding consistency of anions by the effect of aromatic meta-substitution of bis-urea receptors: entrapment of hexafluorosilicate clusters†
Abstract
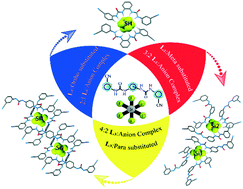
With the cyano group as a terminal substituent, three positional isomeric bis-urea receptors (L1–L3) have been synthesized for extensive analysis of host–anion binding propensity in their neutral form. The existence of electron-withdrawing or π-acidic phenyl substituents in the receptors L1–L3 supports efficient binding of the octahedral hexafluorosilicate anion within their dimeric cavity. L1 and its cyano-substituted para isomer receptor L3 have been found to entrap water-free naked SiF62−, while the meta receptor has been found to trap hydrated SiF62− within its cavity. In the presence of excess fluorides, L1 self-assembles to form dodeca-coordinated hexafluorosilicate complex 1a and L3 self-assembles to form octa-coordinated hexafluorosilicate complex 3a in the solid-state via cooperative and non-cooperative H-bonding interactions of urea moieties respectively. However, the meta receptor L2 self-assembles to build a 3 : 2 host–guest assembly, where the two SiF62− units show different coordination environments. FESEM imaging studies corroborated the result obtained in the solid-state.


 Please wait while we load your content...
Please wait while we load your content...