A pyrazine core-based luminescent Zr(iv) organic framework for specific sensing of Fe3+, picric acid and Cr2O72−†
Abstract
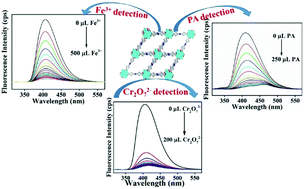
A metal–organic framework (MOF) with a Zr(IV) ion containing a 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine (H4L) ligand was prepared by a solvothermal method. ZrOCl2·8H2O was employed along with the H4L ligand and benzoic acid (modulator) in N,N-dimethylformamide (DMF) to synthesize the title compound. The as-synthesized compound has the formula [Zr6(μ3-O)4(μ3-OH)4(OH)4(H2O)4(L)2]·3H2O·2DMF (1). The activation of 1 was carried out by using methanol exchange and subsequent heating under high vacuum at 130 °C. Both 1 and the activated sample (1′) were characterized by X-ray powder diffraction (PXRD), Fourier transform infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA). They displayed high chemical stability and thermal stability. Both 1 and 1′ are stable up to 440 °C. Compound 1′ has a very high BET surface area (1419 m2 g−E) and CO2 adsorption capacity (4.4 mmol g−1 at 1.4 bar and 0 °C). Being highly water-stable, luminescent 1′ can selectively recognize Fe3+ and dichromate (Cr2O72−) in water and picric acid (PA) in dimethyl sulfoxide (DMSO), by a fluorescence quenching mechanism. The detection limits were found to be 3.75, 13.08 and 8.58 ppb for Fe3+, PA and Cr2O72−, respectively. Moreover, the mechanisms behind this selective detection of all three analytes were also investigated. In all three cases, the compound showed reusability up to five cycles without any loss of sensing efficacy. These experimental data vividly show that 1′ can be considered as a promising sensing material for Fe3+, PA and Cr2O72−.


 Please wait while we load your content...
Please wait while we load your content...