The thermal expansion properties of halogen bond containing 1,4 dioxane halogen complexes†
Abstract
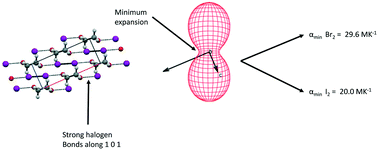
Neutron powder diffraction has been used to determine accurate molecular structures and thermal expansion properties for the complexes formed between by iodine and bromine with 1,4 dioxane. The marked anisotropy in the magnitudes of the principal axes of the thermal expansion tensor for both compounds can be associated with the different intermolecular forces present, with the smallest values being associated with the halogen bonds formed between oxygen and the diatomic halogen molecules. The smallest thermal expansion coefficient of the 1,4 dioxane iodine complex is smaller than the corresponding coefficient in the corresponding bromine complex reflecting the stronger halogen bond formed between iodine and oxygen when compared to that formed between oxygen and bromine.


 Please wait while we load your content...
Please wait while we load your content...