Geometric H/D isotope effect in a series of organic salts involving short O–H⋯O hydrogen bonds between carboxyl and carboxylate groups†
Abstract
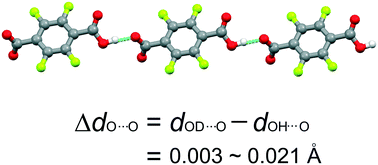
A series of organic salts based on the monoanion of 2,3,5,6-tetrafluoroterephthalic acid were synthesized. All the compounds show characteristic hydrogen-bonded chain structures via short O–H⋯O hydrogen bonds between carboxyl and carboxylate groups. Upon deuteration, the overall structures of these compounds do not change. But the short O–H⋯O hydrogen bonds exhibit noticeable elongations (0.003–0.021 Å) of the O⋯O distance, known as a positive geometric H/D isotope effect. However, there is no definite relationship between the O⋯O distance and the geometric H/D isotope effect due to complex intermolecular interactions between the cations and anions in the crystal structures. Further investigation reveals that there is a weak negative correlation between the O⋯O distance and the packing effect of the cations which can be described by the plane spacing between two adjacent benzene rings of the acid.


 Please wait while we load your content...
Please wait while we load your content...