The high-pressure and low-temperature structural behaviour of 2,2,2-trifluoroethanol†
Abstract
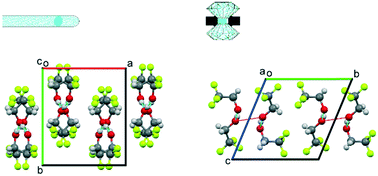
On cooling to 229 K at ambient pressure, 2,2,2-trifluoroethanol freezes to form a crystal structure characterised by hydrogen-bonded molecular chains in the orthorhombic space group Pca21 (form 1). On compression to 0.22 GPa at room temperature, however, liquid 2,2,2-trifluoroethanol crystallises in a structure adopting triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) symmetry (form 2). Although the form 1 and form 2 polymorphs are both characterised by the formation of hydrogen-bonded chains, the arrangement of the molecules within the chains are significantly different with the high-pressure form appearing to adopt a more strained configuration. The orientation of the molecules in form 2 is such that the hydrogen bonds in neighbouring chains are in close proximity with a bridging interchain O⋯O contact distance that is of the same order as the O⋯O distances found for the hydrogen bonds. The resulting close pairing of neighbouring catemeric chains produces molecular pillars in the structure of form 2.
symmetry (form 2). Although the form 1 and form 2 polymorphs are both characterised by the formation of hydrogen-bonded chains, the arrangement of the molecules within the chains are significantly different with the high-pressure form appearing to adopt a more strained configuration. The orientation of the molecules in form 2 is such that the hydrogen bonds in neighbouring chains are in close proximity with a bridging interchain O⋯O contact distance that is of the same order as the O⋯O distances found for the hydrogen bonds. The resulting close pairing of neighbouring catemeric chains produces molecular pillars in the structure of form 2.
- This article is part of the themed collection: The effects of extreme conditions on molecular solids


 Please wait while we load your content...
Please wait while we load your content...