The role of glass crystallization processes in preparation of high Li-conductive NASICON-type ceramics†
Abstract
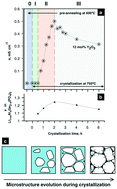
One of the approaches to tackle the problem of limited electrolyte electrochemical stability, which controls the development of novel electrochemical storage devices, is the use of solid electrolytes. Here, we focus on one of the most promising among oxide-based glass-ceramic materials, Li1+xAlxGe2−xP3O12, to investigate the physical and chemical mechanisms that govern the enhancement of Li-conductivity upon variation of its composition and glass crystallization conditions during a two-step heat treatment. Using X-ray and neutron diffraction, small-angle neutron scattering and Raman spectroscopy we found that the addition of 6–18 mol% yttria makes the polyphosphate chains in glass more thermally stable. This leads to sudden and uniform glass crystallization over the whole volume. This peculiar glass structure governs the further crystallization behavior and ensures the optimal organization of intergrain boundaries. The highest Li-conductivity is achieved for a sample with 12 mol% yttria annealed for 2 hours at 750 °C. In addition, nuclear magnetic resonance together with bond valence sum analysis provides further insight into atomistic mechanisms of ionic conductivity in NASICON-based lithium-conductive ceramics.


 Please wait while we load your content...
Please wait while we load your content...