p-Xylylenediamine derived ligands as flexible connectors in the design of porous coordination polymers†
Abstract
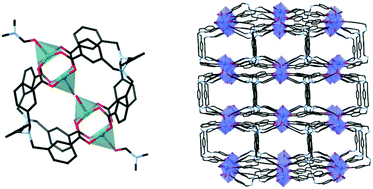
The use of flexible ligands in coordination polymers provides both opportunities and unpredictability. A new series of flexible ligands derived from p-xylylenediamine, which vary in the positioning of carboxylic acid functional groups, have been found to form a series of coordination polymers and a discrete coordination complex. Use of the tetra-substituted meta-carboxylic acid ligand N,N,N′,N′-tetra(3-carboxybenzyl)-p-xylylenediamine, isolated as its dihydrochloride salt (H6M4pxy)Cl2, resulted in the simultaneous formation of two products from the same synthetic conditions. One of these products was a discrete cage-type coordination complex of the form [Cu4(M4pxy)2(DMF)2(OH2)] (1), while the other product was the two-dimensional coordination polymer poly-[Cu2(M4pxy)(OH2)2] (2). Synthesis utilising the tetra-substituted para-carboxylic acid ligand, isolated as N,N,N′,N′-tetra(4-carboxybenzyl)-p-xylylenediamine dihydrochloride (H6P4pxy)Cl2, resulted in the formation of multiple two-dimensional coordination polymers, poly-[Cu2(P4pxy)(DMF)(OH2)]·2DMF·3H2O (3·2DMF·3H2O), poly-[Mn2(P4pxy)(DMA)2] (4), poly-[Co4(P4pxy)2(DMF)2(μ-OH2)2(OH2)2]·6DMF·4H2O (5·6DMF·4H2O) and poly-[Cd2(P4pxy)(OH2)2]·DMA·3H2O (6·DMA·3H2O). The last of these materials, a three-dimensional coordination polymer, was found to adsorb approximately 32 cm3 g−1 STP of CO2 at atmospheric pressure at 273 K.


 Please wait while we load your content...
Please wait while we load your content...