Investigating water vapour sorption kinetics of aluminium MOFs by powder X-ray diffraction†
Abstract
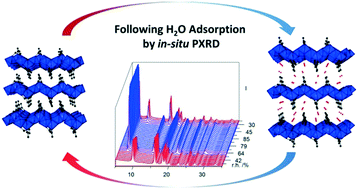
Aluminium isophthalate CAU-10 [Al(OH)(O2C–C6H4–CO2)]·nH2O and aluminium citraconate CAU-15-Cit [Al2(OH)4(O2C–C3H4–CO2)]·nH2O (where CAU stands for Christian-Albrechts-University) were investigated regarding their de- and rehydration behaviour using powder X-ray diffraction (PXRD) and volumetric gas sorption. The relative humidities (r.h.) for ad- and desorption observed by PXRD agree well with the values observed in volumetric experiments. Moreover, the kinetics of hydration and dehydration were followed by PXRD at different temperatures. For CAU-10, the hydration at ≈85% r.h. proceeds much faster than the dehydration at 0% r.h., due to the differences relative to the onset of de-/rehydration at 20% r.h. We could also observe a clear acceleration for both processes between 40 °C and 60 °C. For the layered CAU-15-Cit, the hydration proceeds much slower compared to CAU-10 while the dehydration proceeds faster. This is attributed to the higher hydrophilicity of CAU-10, as well as the associated structural changes during (de-)intercalation in CAU-15-Cit. Eventually, tentative analyses of the kinetics by Sharp–Hancock plots indicate that the sorption processes are in reasonable agreement with (pseudo-) 0th order kinetics.


 Please wait while we load your content...
Please wait while we load your content...