Halide-bi-bridged polymers of amide substituted pyridines and -pyrazines: polymorphism, structures, thermal stability and magnetism†
Abstract
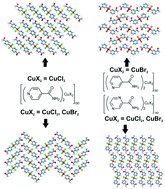
The crystal structures and magnetic properties of seven novel halide-bi-bridged polymers of Cu(II) and the related organic ligands isonicotinamide, nicotinamide and pyrazinamide were determined. Two concomitant, room temperature polymorphs were identified for each of the compounds di-μ2-chlorido-bis(isonicotinamide) copper(II) and di-μ2-bromido-bis(isonicotinamide) copper(II). One of the polymorphs of di-μ2-chlorido-bis(isonicotinamide) copper(II) displays a non-symmetric halide-bi-bridged portion that has never been reported before, the geometry of which significantly affects the magnetic exchange observed in this compound. In addition, the structures of di-μ2-chlorido-bis(nicotinamide) copper(II), di-μ2-chlorido-bis(pyrazinamide) copper(II) and di-μ2-bromido-bis(pyrazinamide) copper(II) are reported. The effect of the change in organic ligand on the resulting hydrogen bonding interactions, and packing of the polymers, as well as the magnetic isolation of the halide-bi-bridged chains is highlighted. Structures and magnetic properties are compared within the family of compounds, and with related compounds reported in the literature, and magneto-structural correlations are identified, while the thermal stability of the compounds is studied via thermogravimetric analysis.


 Please wait while we load your content...
Please wait while we load your content...