Molecular interaction transfer among solvents and solutes modulates the formation of linezolid crystals†
Abstract
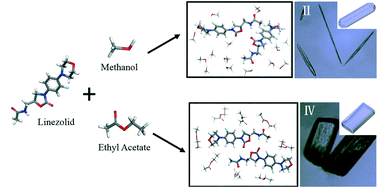
The crystallization of polymorphic linezolid (LZ) was studied via the rapid-cooling method in different solvents to distinguish the relationship between the solvent species and LZ form II versus that of form IV crystals. Then, through molecular dynamics simulation the aggregation behavior of LZ molecules and the time-evolved molecular interaction transfer among solvent and solute molecules were studied in methanol (MeOH) and ethyl acetate (EAC) solvents, respectively. In combination with Hirshfeld surface analysis, it is illustrated that with the EAC solvent there appears interaction transfer from the hydrogen bonds between solute–solvent molecules [N1H(LZ)⋯Oe(EAC)] to those between solute–solute molecules [N1H(LZ)⋯O3(LZ) and CH(LZ)⋯O(LZ)], which facilitates the formation of the LZ form IV crystal. Whereas with the MeOH solvent, there exists interaction transfer from the hydrogen bonds between solute–solvent [OmH(MeOH)⋯O(LZ)] to the solute–solute pairs of [N1H(LZ)⋯O3(LZ) and CH(LZ)⋯π(aromatic-LZ)], which promotes the formation of the LZ form II crystal. In addition, considering the effect of solvents, the crystal habits of LZ form II and form IV were predicted by using the modified attachment energy (AE) model, which reflects somewhat the morphological features of LZ crystals in the solvents MeOH and EAC. The results here disclosed at the molecular level would provide useful guidance on the production of other kinds of polymorphic drugs.


 Please wait while we load your content...
Please wait while we load your content...