Ordered and disordered solvates of C60 and CBrCl2H†
Abstract
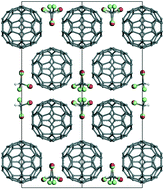
The formation of co-crystals is often unexpected; however, the Buckminster fullerene, for which many solvates are known, is an excellent system to study this tendency. In the present paper, C60 and CBrCl2H solvates have been studied. Hexagonal P6/mmm C60·2CBrCl2H solvates transform at 373 K to monoclinic C2/c C60·CBrCl2H solvates. While orientational disorder typical for C60 is found in the hexagonal solvates (as found in many C60 halomethane solvates), in the monoclinic solvates, C60 appears to be orientationally ordered. As for the solvent CBrCl2H, two types of occupational disorder involving the distribution of the halogen atoms can be observed, comparable to the behaviour in the two polymorphs of pure CBrCl2H. Within the monoclinic solvate, two sites are equally occupied by Br and Cl atoms (1/2 : 1/2), while one site is fully occupied by a Cl atom which leads to an average Cs symmetry for the solvent molecule. Whereas within the hexagonal solvates, each halogen position is occupied by 1/3 : 2/3 of Br or Cl atoms, respectively, leading to an average C3v molecular symmetry. The anisotropy of the intermolecular interactions coincides with the symmetry of the solvate structures and can be generalized for the C60·halomethane solvates.


 Please wait while we load your content...
Please wait while we load your content...