Orientation-dependent conformational polymorphs in two similar pyridine/pyrazine phenolic esters†
Abstract
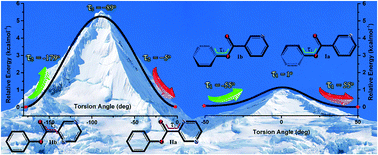
On the basis of crystal engineering, two similar esters of phenyl pyridine-2-carboxylate (I) and phenyl pyrazine-2-carboxylate (II) were designed and synthesized. The compounds were characterized using FT-IR, mass spectrometry, CHN-elemental analyses, NMR and PXRD. For each compound, two polymorphs were obtained (Ia, Ib and IIa, IIb) and identified by TGA, DSC and SCXRD. A comparison of the crystal structures of the polymorphs revealed that the different torsion angles of the pyrazine (τ1) and phenyl (τ2) rings to the ester backbone resulted in the conformers I and II, respectively. Theoretical criteria (max(Δθ), rmsd[r]-crystal, energy profile) confirmed that the structural differences in the conformers are in the range of acceptable values for the detection of conformational changes. The phase stability of the polymorphs was investigated by slurry and grinding methods as well as by the HSM technique. Since the orientations of pyrazine and phenyl moieties were altered in the polymorphs, the hydrogen bond donor and acceptors exhibited meaningful supramolecular architectures in the crystal packing as well as C–H⋯N, C–H⋯O and C–H⋯π interactions. The energetic study of the noncovalent interactions in the molecular pairs (dimers) of the polymorph crystal structures was performed by DFT-D calculations.


 Please wait while we load your content...
Please wait while we load your content...