Molten salt synthesis, growth mechanism, and photoluminescence of rod chlorapatite microcrystallites†
Abstract
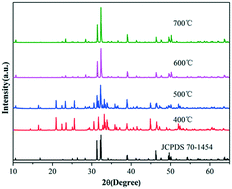
Innovative syntheses of uniform and size-controlled chlorapatite crystals need to be developed. Molten salt synthesis is a simple process, which uses low temperatures and short reaction times to obtain synthesized powders with uniform chemical composition as well as good crystal morphology and high-phase purity. In this study, a molten salt synthesis was used to prepare ClAP microcrystallites at different temperatures, reaction times, and quantities of molten salt. ClAP microcrystallites with different sizes and morphologies were obtained, and the process parameters were optimized. The phase formation and morphologies of the ClAP microcrystallites were examined by X-ray powder diffraction and scanning electron microscopy. The results determined the optimal parameters, as follows: synthesis temperature, 600 °C; reaction time, 4 h; and ClAP/molten salt ratio, 1 : 3. The reaction time and the quantity of the molten salt, as well as the growth mechanism of the ClAP microcrystallites, were proposed based on the phase formation and morphologies of ClAP synthesized at various temperatures. ClAP phosphors activated using different ions (Sb3+, Eu2+, and Tb3+) were prepared by the molten salt method at 600 °C for 4 h with a ClAP/salt ratio of 1 : 3. The as-prepared ClAP:Sb3+/Eu2+/Tb3+ phosphors exhibited a blue-green emission (470 nm), a blue emission (460 nm), and a green emission (547 nm). Their excellent photoluminescence properties indicated their potential for application in white light emitting diodes.


 Please wait while we load your content...
Please wait while we load your content...