Cycloalkanes and cycloalkenes in dispersive force oriented inclusion crystals by a functionalized acyclic host molecule†
Abstract
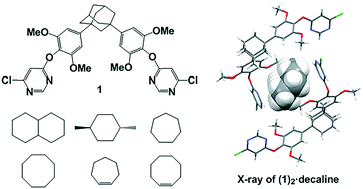
A disubstituted adamantane derivative bearing chloropyrimidine units (1) was synthesized as an acyclic host molecule to construct guest-inclusion crystalline materials, and the molecular structures of these crystalline materials were determined by X-ray crystallographic analysis. The crystallization of 1 and trans-decahydronaphthalene (a) in chloroform afforded co-crystal (1a) with 2 : 1 host : guest complexation stoichiometry. The guest molecule was accommodated within the cavity of the cyclic dimers of 1 built from Cl⋯π interactions between the chloropyrimidine units. These complexes were assembled into network structures through intermolecular interactions. From the Hirshfeld surface analysis and the 2D fingerprint plot of a in co-crystal 1a, the maximum contribution is from the H⋯H contacts between 1 and a, indicating that the main driving force for the guest inclusion is dispersive force. Co-crystals containing cycloalkanes and cycloalkenes (b–f) and having the same stoichiometry were obtained under identical conditions. In all the co-crystals, the shapes and sizes of the cyclic dimers and the molecular networks were similar to those of crystal 1a, and the molecular structures of the guests were characterized.


 Please wait while we load your content...
Please wait while we load your content...