O(−)⋯C interactions and bond formation in 1-naphtholate anions with peri-located electrophilic carbon centres†
Abstract
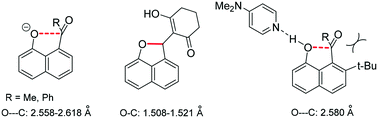
The first peri interactions between naphtholate oxyanions and electrophilic double bonds are described. Tetramethylguanidine forms crystalline salts with 8-acetyl- and 8-benzoyl-naphthol which show O⋯C distances for the anions in the range 2.558–2.618 Å and small increases in carbonyl pyramidalities over the corresponding naphthols, whereas DMAP forms only hydrogen bonded complexes. Replacement of the acyl group with an alkene leads to intramolecular O–C bond formation for just the most electron deficient alkenes, with long peri O–C bonds (1.508 and 1.521 Å) observed in one case. However, both cyclised and uncyclised examples can be deprotonated to give cyclic structures according to NMR, and DFT calculations suggest very long peri-O–C bond lengths of 1.540 and 1.622 Å for two of these anions.


 Please wait while we load your content...
Please wait while we load your content...