Crystallographic studies of 2-picolyl substituted naphthalene diimide and bis-phthalimide ligands and their supramolecular coordination chemistry†
Abstract
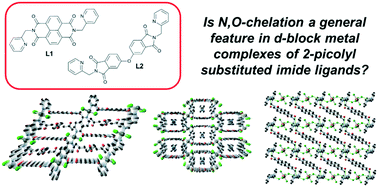
Here we report the synthesis of two N-(2-picolyl) substituted bis-imide ligands, N,N′-di(2-picolyl)-1,4,5,8-naphthalenetetracarboxylic diimide L1 and N,N′-di(2-picolyl)-4,4′-oxybisphthalimide L2, and describe their coordination chemistry in the crystalline state with late d-block metals, with the intention of probing the applicability of the recently reported N,O-chelating mode observed in N-(2-picolyl) substituted 1,8-naphthalimides. Four new crystalline coordination compounds have been prepared and structurally characterised; poly-[Zn(L1)Cl2]·3(C3H6O) 1 and poly-[ZnCl2(L1)]·MeCN 2 are structurally related one-dimensional coordination polymers whose extended structure contains well-defined solvent channels, the nature of which is coupled to the ability of the lattice solvent molecules to undergo n⋯π interactions with the 1,4,5,8-napthaletetracarboxylic diimide (NDI) core. [H2L1][ZnCl4]·2H2O 3 is a hydrogen-bonded structure of tetrachlorozincate anions bound by the doubly protonated H2L1 cation, while repeating this reaction in the presence of copper(II) ions gave the ligand dihydrochloride salt (H2L1)2Cl 4. Finally, reaction of L1 with AgSbF6 gave the one-dimensional polymer poly-[AgL1]SbF65, in which weak but notable carbonyl coordination was observed in addition to stronger coordination from the pyridyl groups. Conversely, compound L2 failed to convincingly show any reaction or coordination with transition metals, and only the crystalline ligand itself could be isolated. Analysis of these results, as well as studies into the solution state coordination chemistry of these compounds, suggests an underlying barrier to coordination in these species compared to the 1,8-naphthalimides, but provides interesting avenues for crystal engineering.
- This article is part of the themed collection: Introducing the CrystEngComm Advisory Board and their research


 Please wait while we load your content...
Please wait while we load your content...